Neonatal anesthesia and its developmental neurotoxicity
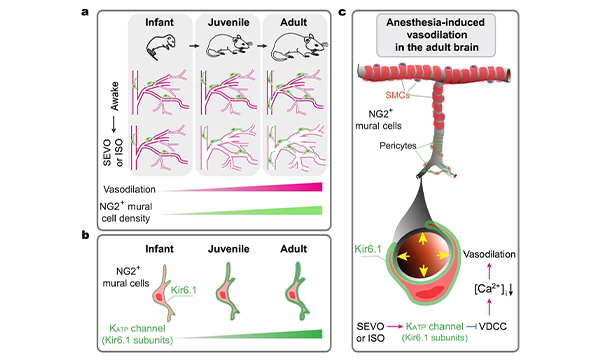
Age-dependent cerebral vasodilation induced by volatile anesthetics is mediated by NG2+ vascular mural cells
Anesthesia can influence cerebral blood flow by altering vessel diameter. Using in vivo two-photon imaging, we examined the effects of volatile anesthetics, sevoflurane and isoflurane, on vessel diameter in young and adult mice. Our results show that these anesthetics induce robust dilation of cortical arterioles and arteriole-proximate capillaries in adult mice, with milder effects in juveniles and no dilation in infants. This anesthesia-induced vasodilation correlates with decreased cytosolic Ca2+ levels in NG2+ vascular mural cells. Optogenetic manipulation of these cells bidirectionally regulates vessel diameter, and their ablation abolishes the vasodilatory response to anesthetics. In immature brains, NG2+ mural cells are fewer in number and express lower levels of Kir6.1, a subunit of ATP-sensitive potassium channels. This likely contributes to the age-dependent differences in vasodilation, as Kir6.1 activation promotes, while its inhibition reduces, anesthesia-induced vasodilation. These findings highlight the essential role of NG2+ mural cells in mediating anesthesia induced cerebral vasodilation. (Commun Biol, 2024)
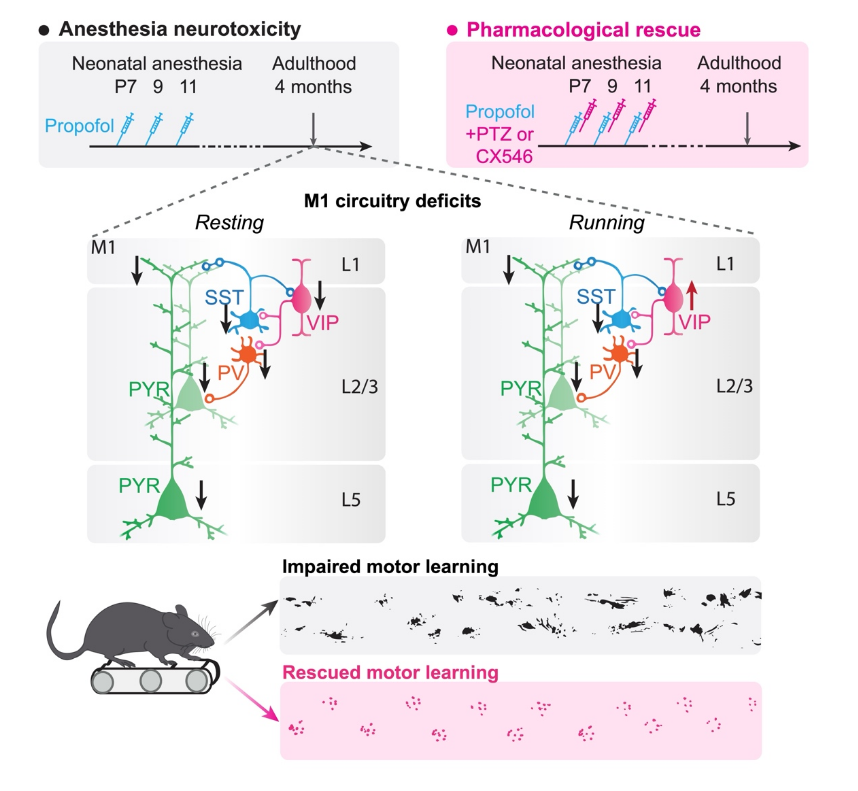
Behavioral impairments after early life propofol exposure are accompanied by reductions in neuronal activity in cortical circuitry
Both animal and retrospective human studies have linked extended and repeated anesthesia during early development with cognitive and behavioral deficits later in life. However, the neuronal circuit mechanisms underlying this anesthesia-induced behavioral impairment are poorly understood. Neonatal mice were administered one or three doses of propofol, a commonly used general anesthetic, during postnatal days 7 to 11. Control mice received intralipid injections. At 4 months of age, mice were subject to a series of behavioral tests including motor learning. During the process of motor learning, calcium activity of pyramidal neurons as well as three classes of inhibitory interneurons in the primary motor cortex were examined in vivo using two-photon microscopy. Repeated, but not a single, exposure of neonatal mice to propofol caused motor learning impairment in adulthood, which is accompanied by a reduction of pyramidal neuron number and activity in the motor cortex. The activity of local inhibitory interneuron networks was also altered: somatostatin-expressing and parvalbumin-expressing interneurons were hypoactive, whereas vasoactive intestinal peptide-expressing interneurons were hyperactive when mice were performing a motor learning task. Administration of low dose pentylenetetrazol to attenuate GABAA receptor-mediated inhibition or CX546 to potentiate AMPA receptor function during emergence from anesthesia ameliorated neuronal dysfunction in the cortex and prevented long-term behavioral deficits in mice. Our results reveal that repeated exposure to propofol anesthesia during early development causes cortical circuit dysfunction and behavioral impairments in later life. Potentiation of neuronal activity during anesthesia recovery reduces these adverse effects of early-life anesthesia in mice. (Brit J Anaesth, 2021)